It also describes how likely it is to find the electrons in various locations around an atoms nucleus. Protons and neutrons form the atomic nucleus.

Electron Cloud Model Of Atom Basic Laser Principles Laser It Technologies Quantum Mechanics Quantum Physics Quantum Entanglement
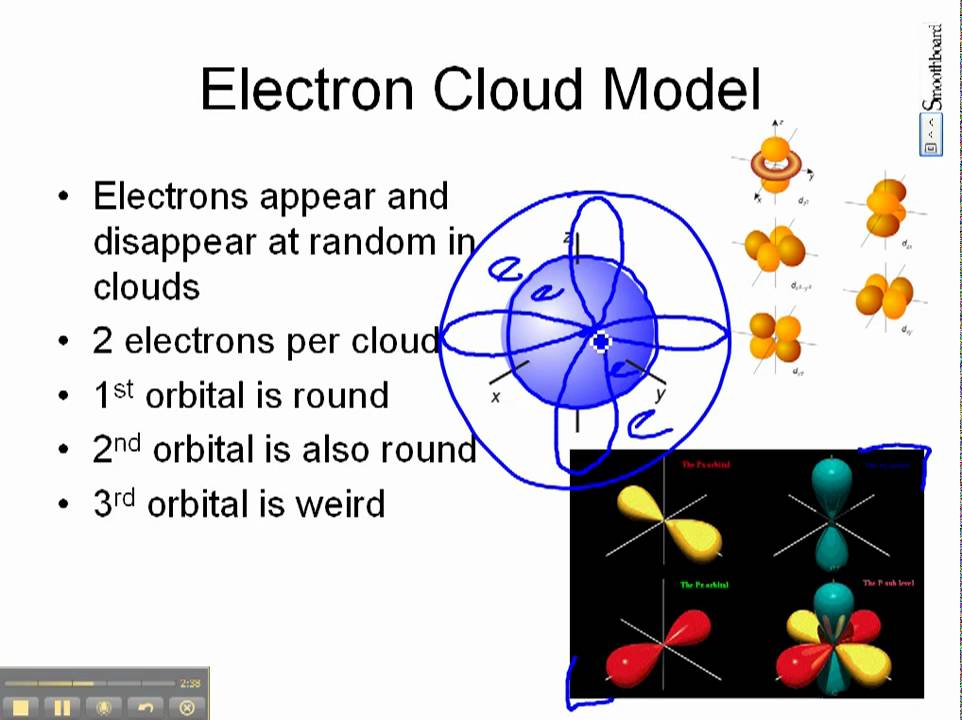
The Electron Cloud model.

. How does floor 2 of Hotel Krypton describe all atoms electron configuration. Describe what is meant by the electron cloud model of the atom it that arises from schrodingers wave equation. The shape not the size of an electron cloud is determined by the electrons ____.
Which of these statements describe the two models correctly. In the electron cloud model of the atom what does the cloud represent. Electron cloud is an informal term in physics.
Describe the main difference between the Bohr model and the electron cloud model of the atom Include. Bohr proposed that an electron exists only in specific circular paths or orbits around the nucleus. Similarly you may ask why is it called the electron cloud.
The electron cloud model describes the of electrons in an atom. Describe exactly where the electrons are around the nucleus b. The electron cloud is a cloud of probability surrounding the nucleus in an atom where one has the highest probability of finding an electron.
In the cloud model there are regions where an electron may likely be found but its theoretically possible for it to be located anywhere including inside the nucleus. The electron cloud model differs from the more simplistic Bohr model in which electrons orbit the nucleus in much the same way as planets orbit the sun. Bohrs model electrons cannot exist between orbits but in the electron cloud model the location of the.
Check all of the boxes that apply. The electron cloud refers to a region outside the nucleus where an electron is most likely to be found. What is the current accepted theory of the atom.
The probability of the electron location. You might also like to know about the parts of an atom before. Bohr talked about electrons orbiting the nucleus.
The Electron Cloud Model Of An Atom Till Lindemann Metal Wings. Describe what is meant by the electron cloud model of the atom it that arises from schrodingers wave equation. Determine the number of electrons in a certain energy level d.
The electron cloud model describes the of electrons in an atom. Chemistry 22062019 0200 biggz8232. Electron Cloud Model.
The quantum mechanical model describes the allowed energies an electron can have. What is the electron configuration of this atom. Likewise whats the electron cloud model.
All elements have an s and p subshell available for electrons in level 2. It is used to describe where electrons are when they go around the nucleus of an atom. The electron cloud model is a visual representation of the possible locations of electrons in an atom.
Predict the weather c. An cloud atom model of electron the. Click to see full answer.
In the Bohr model an electrons position is known precisely because it. The electron cloud model was developed in 1925 by Erwin Schrödinger and Werner Heisenberg. 1s2 2s2 2p6 3s2 3p6 4s1.
The electron cloud is used to describe the behavior of electrons and it is useful in building a model of the atom. The model is an approach to help picture the most likely position of electrons in a molecule. Bohrs model was replaced only because of its age.
The electron cloud model of the atom is used to do which of the following. Erwin Schrodinger an Austrian physicist came up with the electron cloud model in 1926. When you think of an atom your min d probably conjure s up an image of a central nucleus with a.
1221 students attemted this. The term electron cloud was coined by an American physicist named Richard Feynman. The electron cloud model best describes the organization of electrons around the nucleus of an atom.
The electron cloud shows the area in space where an electron is most likely to be. Model of the Atom. The electron cloud model is at present the acknowledged model of an atom.
The electron cloud model describes the _____ of electrons in an atom. Describe the possible locations of electrons around the nucleus. Before understanding what an electron cloud model is it is important to know about the forces that bind the electrons together.
For the atom of krypton there are eight total electrons in level 2. In this model of the hydrogen atom you have a black screen and then you draw a. The electron cloud model describes the _____ of electrons in an atom.
The electron cloud model was created in 1926 by Werner Heisenberg and Erwin Schrödinger. 1 Get Other questions on the subject. The electron cloud model is the current accepted model of an atom.
The electron cloud model is different from the older Bohr atomic model by Niels Bohr. Smenevacuundacy and 7 more users found this answer helpful. Which statement about the electron-cloud model is true.
A spherical electron cloud surrounding an atomic nucleus would best represent. Nuclear reactions can alter atoms. The model is a way to help visualize the most probable position of electrons in an atom.
Which of these describes the electron behavior necessary for sodium to bond with another element. The three parts of the atom are protons positively charged neutrons neutral charge and electrons negatively charged. Compare the modern electron cloud model of the atom with Bohrs atomic model.
Intersects With Rectangular Pools. An atom is a building block of matter that cannot be broken apart using any chemical means. There are two electrons in the s subshell and six electrons in the p subshell.
The Electron Cloud Model was of the greatest contributions of the 20th century leading to a revolution in physics and quantum theory. If you add 10ml of hot water to 10ml of cold water and the.

Electron Cloud Model Electrons Elementary Science Clouds

The Electron Cloud Model Explained Animation Atomic Orbitals Tutorial Crash Chemistry Academy Youtube Chemistry Worksheets Chemistry Help Astrophysics
0 Comments